Project organization
& workpackage
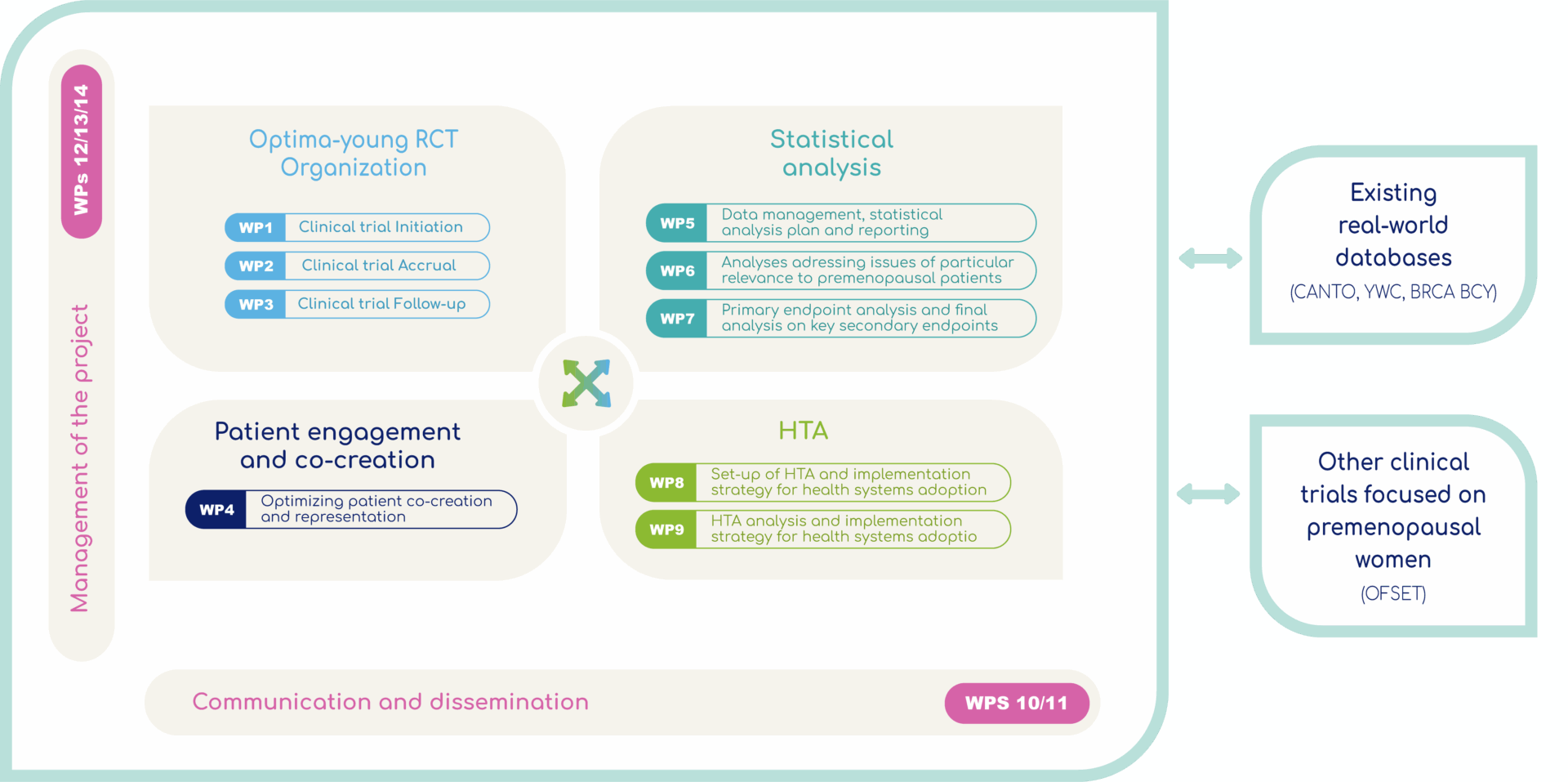
WP1-3 Optima-Young RCT Organization
WP1-3 Optima-young Organization: Two twin trials are associated with Path-for-Young: Optima-young sponsored by UNICANCER conducted on pre-menopausal patients and OPTIMA sponsored by University College of London (UCL) operated by University of Warwick conducted on pre-menopausal and post-menopausal women. Both Optima-young and OPTIMA are international, randomized, two arm clinical studies to verify wheter the use of a genomic test (Prosigna®) to decide whether or not to administer chemotherapy gives results as good as standard treatment (systematic chemotherapy) in premenopausal women with hormone-dependent (HR+)/HER2- breast cancer, by assessing their risk of cancer recurrence.
- Multiparameter gene expression assay: Several tests have been developed to study the genes present in breast cancer cells. These tests measure the degree of activity of some of these genes. They are used to guide and improve the choice of treatments by providing precise information about each cancer. These tests are sometimes called multiparametric tests.
- Digital platform WeShare: The Path-for-young project deploys a dedicated digital infrastructure and data collection web platform (WeShare), leveraging decentralized collection of longitudinal patient-generated-data to evaluate patient quality of life and key toxicities.
Access to WeShare Website - Digital Health Tools: A substudy of Optima-young RCT, which will formally test, among participating patients from 3 European countries a digital
companion to deliver enhanced follow-up care providing care patients with a personalized supportive care pathway to manage side effects associated with endocrine therapy to maximize quality of life, self-efficacy and empowerment, and clinical outcomes via improved treatment adherence link to Resilience.
WP 4 Patient engagement and co-creation
The project practices co-creation with multiple stakeholders, primarily patients and HCP with relevant experience, throughout the entire duration of the project beginning during protocol development, to enhance trial recruitment and implementation as well as ensuring that key patient needs are integrated into integratedto the trial development and conduct. link to section patient engagement.
WP5-7 Statistical anaylsis
This work package will analyze the data collected during the Optima-young study :
All the tasks related to planning of the statistical analyses of the Optima-young RCT, as well as the first interim analysis to study robustness of the initial study hypotheses will be done within the work package 5. The main statistical analysis and reporting of the clinical trial, and the analyses of secondary endpoints using the last data lock at the end of the clinical trial among all enrolled patients will be done within the work package 7.
WP 6 The work package 6
The work package 6 will ensure that the first analyses of the digital companion substudy, which will test the effectiveness of existing personalized digital health tools to address issues of particular relevance to premenopausal patients in a personalized supportive care pathway. In addition, the WP will have the objective to evaluate issues of relevance to premenopausal patients, and of the societal impact of the Optima-young RCT personalized approach.
WP 8-9 HTA
The aim is to assess the efficiency and budgetary impact of the use of the genomic test to guide the prescription of the chemotherapy and to prepare the health system to the adoption of the new strategy.
- the work package 8 will describe the current use of technology and technical characteristics across the international HTA partners; plan a cross-country cost and economic effectiveness to develop a methodology ensuring transferability to all EU countries and will perform an ethical analysis, and do analysis including organisational, patient, social, and legal aspects;
- the work package 9 will deliver the Health Technology Assessment including the health economic analyses and to ensure that the Optima-young RCT findings are implemented in the real-world;
WP 10- 11 Communication
A dedicated work package will work on the communication of the project to raise awareness of the project among the general public and the scientific community through publications on social networks and in medical networks.
WP12-14 Management of the project
The aim of this work package is to coordinate all the parties involved in the project to ensure quality and the achievement of objectives within the allotted timeframe